Hi, my name is Li Zehua, a master students of Advance Functional Materials at the University of Glasgow, I recently completed my practical project in the Mehr research group.

During my undergraduate studies, I developed a strong interest in organic chemistry. My main focus was on alkali metal catalysis and photo-induced phase change energy storage dyes, primarily using solvent-based methods to synthesize reactants and explore their reaction mechanisms— a process that was quite “traditional”. When I joined Hessam’s lab, I encountered parallel aerosol reactors for the first time. I was amazed by this novel method of synthesis and decided to experiment with synthesizing known compounds using the aerosol reactor to test its potential in compound synthesis.
Due to the exceptionally high surface area to volume ratio of droplets in aerosols, substances can more rapidly interact with the heat source or reaction environment, thereby accelerating heat transfer and reaction rates. This means that chemical reactions can be effectively promoted even at lower temperatures. In addition, the evaporation of the solvent is an endothermic process, which allows the aerosol droplets to locally increase their temperature during evaporation without the need for an external heat source. These properties make aerosol methods particularly suitable for synthesis reactions that require mild heating or low thermal initiation.For instance, in the synthesis of macrocyclic Schiff bases from aldehydes/ketones and amines, the dehydration step involved not only stabilizes the product by forming double bonds but also releases heat, providing the necessary activation energy for the reaction. This is beneficial for synthesizing longer chains or even [3+3] type macrocyclic Schiff bases. Consequently, we chose macrocyclic Schiff bases as our target products for synthesis.
What is a Schiff base?
Schiff bases are a type of chemical compound that often come up in organic chemistry. They’re made when an amine (that’s a molecule containing nitrogen bonded to three different organic groups) reacts with an aldehyde or ketone (these are types of compounds that have a carbonyl (C=O) group, which includes a carbon atom double-bonded to an oxygen atom). When they react, they form a new compound called an imine or Schiff base featuring a double bond between a nitrogen and a carbon atom. This reaction is pretty simple but super important because Schiff bases can be used in lots of different ways, like in dyes, pigments, and even some medicines.
Macrocyclic Schiff bases are like the bigger, more complex cousins of regular Schiff bases. The “macro” part of the name means large, and “cyclic” means ring-shaped, so these are large ring-shaped molecules. They also have the characteristic nitrogen-to-carbon double bond, just like regular Schiff bases. What makes them special is their size and structure, which allow them to form very stable complexes with metals. This makes them useful in more advanced applications, like catalysis (which helps speed up chemical reactions), or in creating materials that can grab and hold onto specific molecules, a bit like how a lock holds a key. This can be really handy in areas like environmental science, where you might want to capture pollutants, or in medicine, for delivering drugs to specific parts of the body.
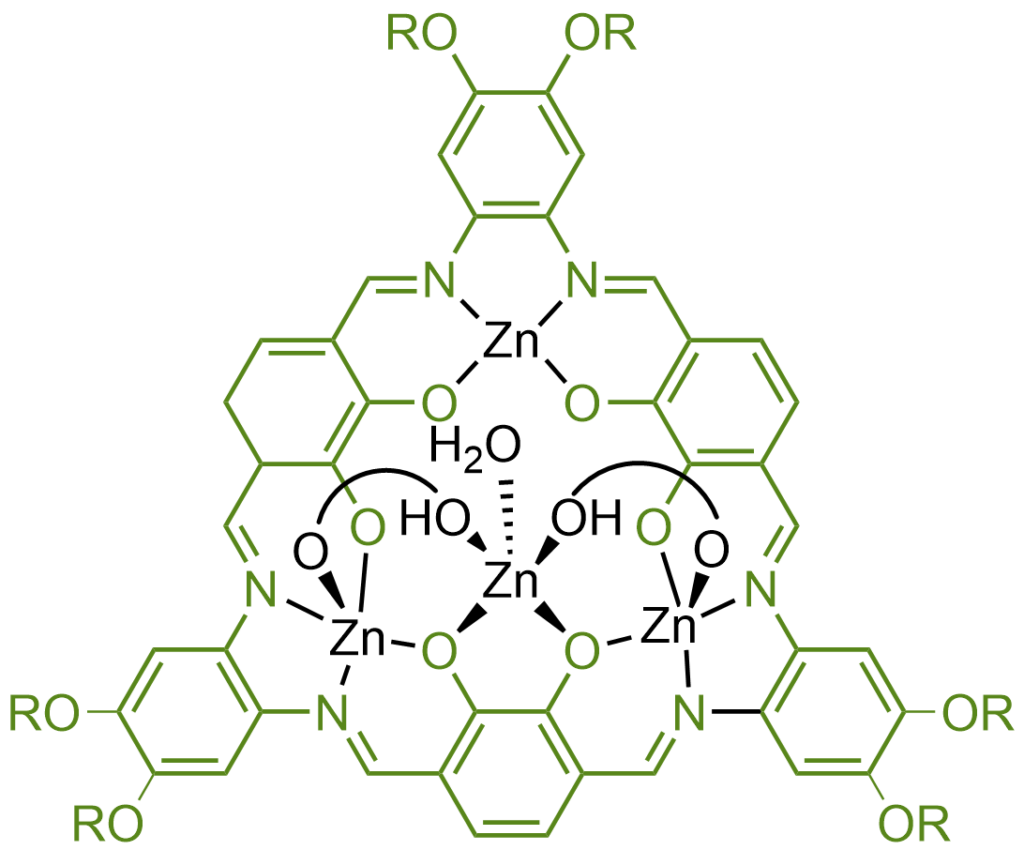
What are aerosols and why use them in our experiments?
Aerosol synthesis is an innovative chemical synthesis technique that suspends reactants as extremely fine particles in a gas, with the goal of enhancing reaction efficiency and product quality. This method stands out compared to traditional solution-based approaches for several reasons. First, the reactants in aerosol form have a much larger surface area, which may speeds up reactions and increases their progress towards completion. This makes aerosol synthesis particularly suitable for chemical reactions that require fast reactivity and high yield.
Another major advantage of aerosol synthesis is its environmental friendliness. Traditional chemical synthesis often involves large amounts of organic solvents, which are not only costly but also pose significant environmental risks. Aerosol technology can significantly reduce or even eliminate the use of solvents, thereby lowering environmental impact and reducing energy consumption. For instance, in the production of nanomaterials, aerosol methods can avoid the use of organic solvents at high temperatures, reducing the emission of harmful substances.
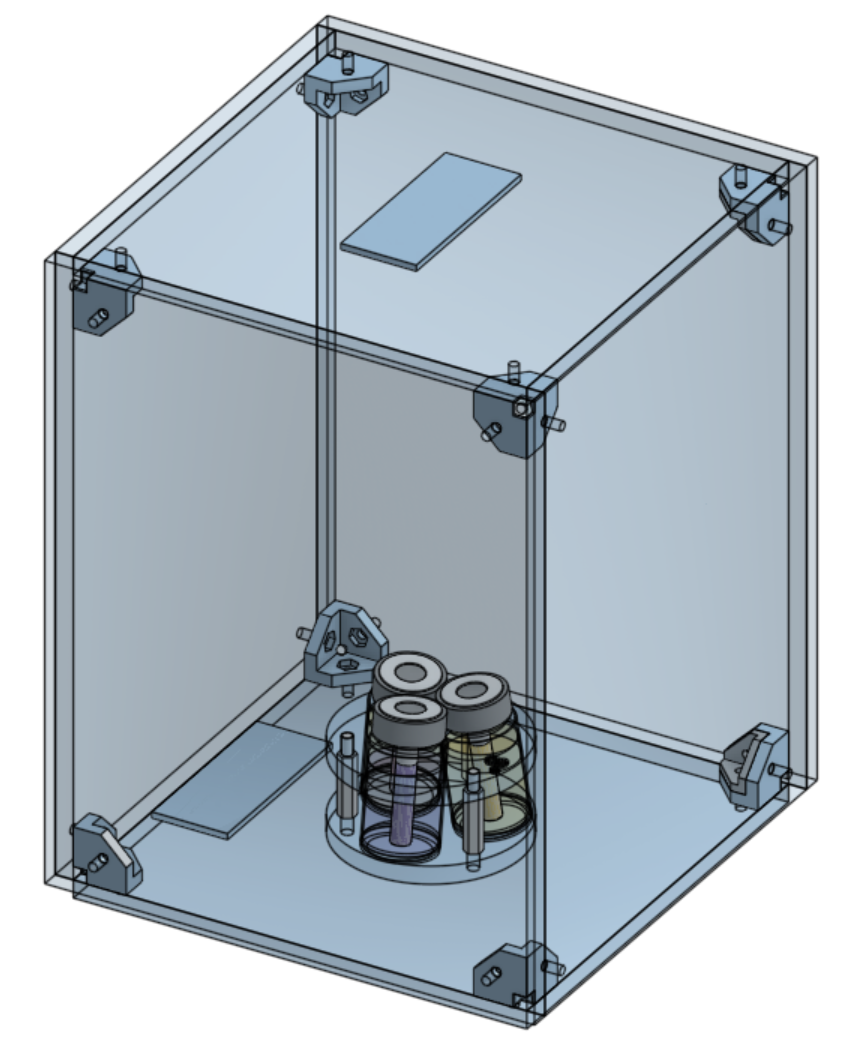
Despite its many benefits, aerosol synthesis does have some limitations. For example, it requires specialized equipment to generate and control aerosol particles, which means higher technical requirements and initial investments. Additionally, maintaining the stability of aerosols and precisely controlling particle size are technical challenges that need to be addressed in experiments. However, with ongoing technological advancements and further research, aerosol synthesis is showing tremendous potential in fields like pharmaceuticals, catalyst fabrication, and high-performance materials synthesis. As technology continues to advance, it is expected that aerosol synthesis will play an increasingly important role in chemical manufacturing, especially in an era that emphasizes green and sustainable chemical processes.
How did we approach the problem?
We started by searching for reaction formulas related to the synthesis of macrocyclic Schiff bases using the traditional bulk solution method, selecting reaction systems that are mild, with temperatures slightly above or equal to room temperature. Next, we replicate these reactions using the original published protocol to make sure they are reproducible. Once verified successfully, we then attempt to synthesize the macrocyclic Schiff bases using aerosol synthesis in our aerosol reactor.
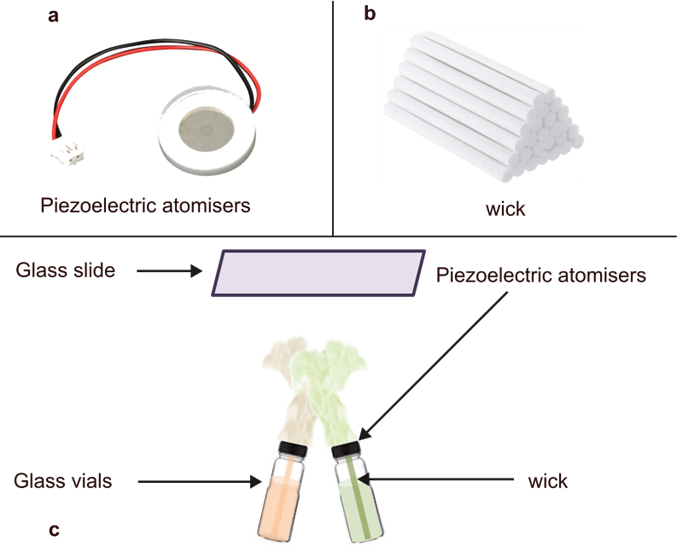
To create macrocyclic Schiff bases using an aerosol reactor, we prepared two reagent vials. In one, we dissolved the amine in solvent; in the other, we dissolved aldehyde also in solvent. We also added a small amount of acid to the aldehyde mixture because acid can speed up or catalyze Schiff base formation.
Next, we fitted each reagent vial with a piezoelectric element, whose role is to convert the solution into a mist of aerosol particles, and placed the vial assembly inside the aerosol reactor. Finally, we connected the reactor to a computer which is programmed to control the emission aerosol by activating the piezoelectric elements.
What is a piezoelectric actuator?

A piezoelectric atomizer is a small device that uses quick vibrations to turn liquids into a fine mist. It works by using a special material that vibrates rapidly when electricity is applied, creating pressure waves that break the liquid into tiny droplets. You can find these atomizers in everyday items like humidifiers, which add moisture to the air, aroma diffusers, which spread essential oils, and medical devices like nebulizers, which help people inhale medicine. They’re popular because they can create a fine mist without using heat, making them efficient and safe for delicate liquids.
By positioning the vials at a slight angle relative to each other, we encouraged microdroplets from each source to collide with each other to form the Schiff base. We collected the products of aerosol reaction on two glass slides within the reactor, one at the top and one at the bottom. This setup allowed us to capture and analyze the products separated afterwards.
After the Schiff base reaction is completed, the amine (-NH2) and aldehyde (-C=O) groups undergo a condensation reaction to form an imine bond (-C=N-). Therefore, we can use infrared spectroscopy to detect functional groups in the product and look for the (-C=N-) absorption peak, which indicates the presence of the imine bond. After calculating the theoretical molecular weight of the target product, we also used mass spectrometry to measure the mass-to-charge ratio of the reaction product, from which we can infer the actual mass of the product. By comparing the measured mass with the calculated mass, we can verify whether the molecule obtained is the target product. These steps help us to get a better idea of which product molecules have formed.
What is infrared spectroscopy?
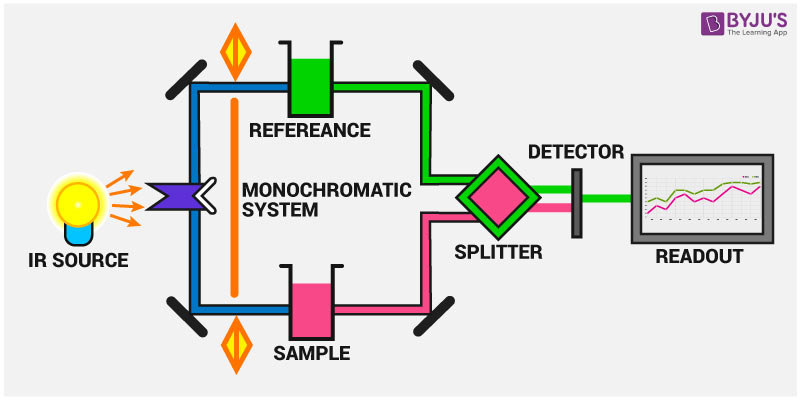
Picture source(https://byjus.com/chemistry/infrared-spectroscopy/)
Infrared spectroscopy (IR) is a practical chemical analysis technique that identifies and studies chemical substances by measuring specific frequencies at which compounds absorb infrared light. Each chemical has its unique “molecular fingerprint,” consisting of a series of specific absorption peaks that reflect the vibrations of chemical bonds within the molecule. This technique allows us to quickly determine the type and molecular structure of substances and is widely used in research, quality control, and environmental monitoring. The advantages of IR spectroscopy include its speed and non-destructive nature, providing accurate chemical information without damaging the sample.
What is Mass spectroscopy?
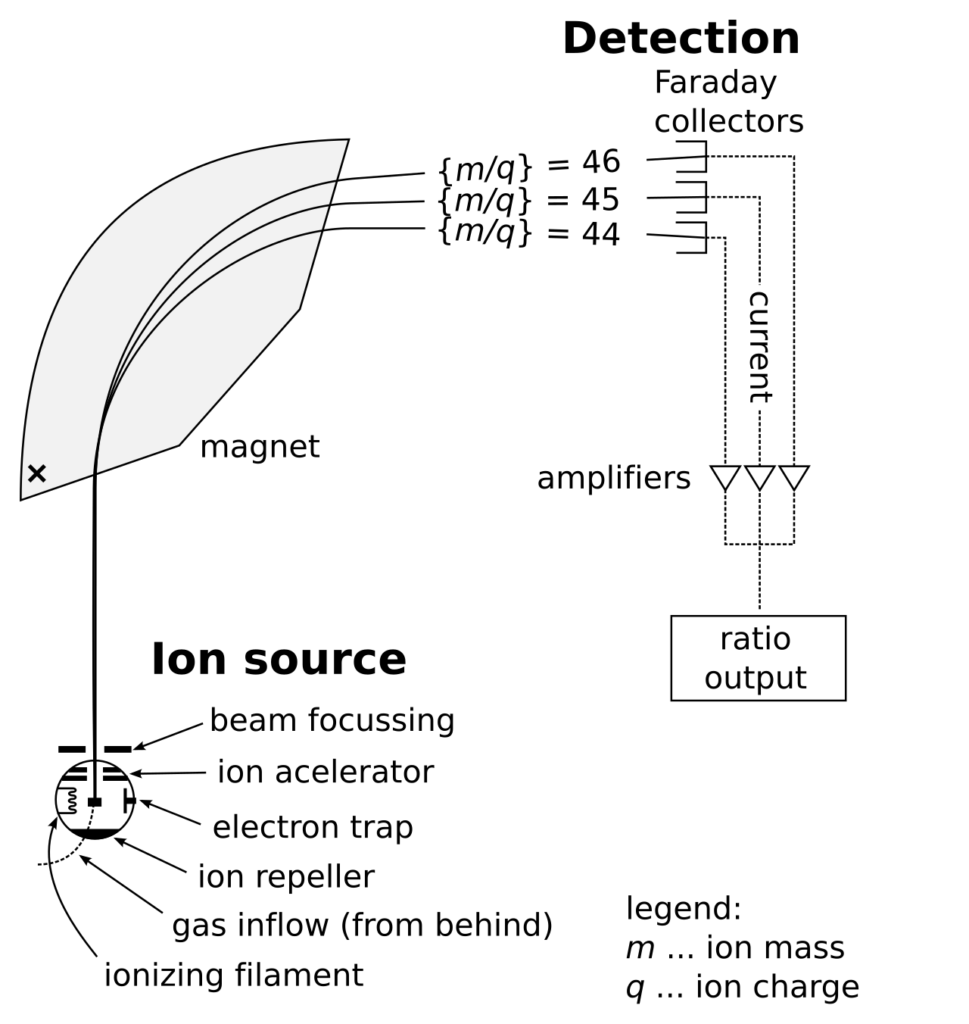
Picture source
https://en.wikipedia.org/wiki/Mass_spectrometry#/media/File:Mass_Spectrometer_Schematic.svg
Mass spectrometry (MS) is an analytical technique used to identify compounds and measure their abundance by analyzing the mass-to-charge ratio (m/z) of sample molecules. In this process, the sample is first ionized to create charged particles, which are then accelerated in an electromagnetic field and separated based on their mass-to-charge ratios. A mass spectrometer generates a spectrum by detecting these separated ions, showing the distribution of ions with different m/z values, which helps to identify the chemical substances and their structures within the sample.
The applications of mass spectrometry are vast and include drug development, biological research, environmental science, and forensic science. The main advantages of this technique are its high sensitivity and the ability to detect trace amounts of substances, providing precise molecular mass information. Mass spectrometry can also analyze multiple components in complex samples, making it an indispensable tool in modern analytical science.
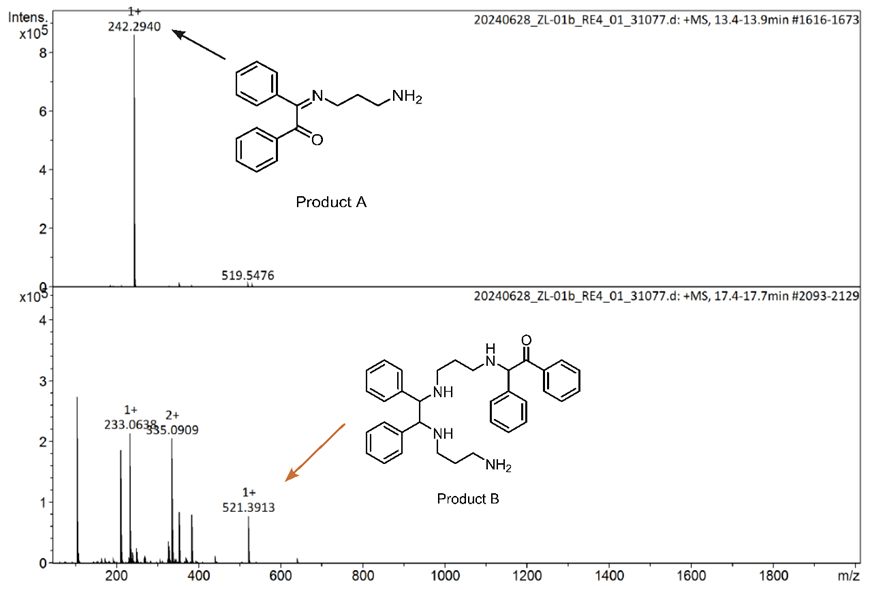
Future plan
This study primarily showcases an innovative synthesis technique—using aerosol chemistry to manufacture macrocyclic Schiff bases. Although macrocyclic Schiff bases themselves have been extensively studied, the highlight of this research is the development of a brand new method of preparation. This method, which synthesizes through the collision of micron-sized aerosol droplets, not only enhances synthesis efficiency but also reduces environmental impact, offering a sustainable and efficient option for the field of chemical manufacturing.
In the future, we can focus on further refining this technology by introducing electrodes to enhance interactions between aerosol droplets using electrostatic forces to improve the success rate of reactions. Additionally, adjusting the boiling point of solvents and precisely controlling reaction temperatures will enhance reactivity and reduce unwanted side reactions, increasing the quality and yield of products.
These improvements will not only provide an opportunity to deeply understand the mechanisms of aerosol chemical reactions but also suggest that this new method could transform the future of chemical manufacturing. With this innovative synthesis approach, we look forward to opening a new chapter in chemical synthesis, especially in terms of efficient and environmentally friendly synthesis strategies.

Over the past three months, I have had the privilege of being guided by Dr. Hessam Mehr, which has allowed me to rediscover the field of organic synthesis from an aerosol perspective. Without his guidance, I would not have been able to complete this complex project. Dr. Mehr’s extensive knowledge in organic chemistry, supramolecular chemistry, and software programming has been immensely beneficial to me. His expertise not only helped me address various challenges in the laboratory but also greatly broadened my academic horizon. Working with him has been a highly enjoyable and fruitful experience, deepening my understanding of chemical research.
I sincerely thank Dr. Hessam Mehr for his meticulous guidance and selfless assistance, and I hope to have the opportunity to collaborate with him again in the future. I would also like to express my gratitude to the other members of the Hessam group. I am particularly thankful to Zhang Luokun for his significant help with modeling the vial holder, Ms. Mariam Kalathil for her support in carrying out the experiments, and fellow student Chen Junyi for his valuable writing advice. Their assistance has been crucial to my research work.

Leave a Reply
You must be logged in to post a comment.